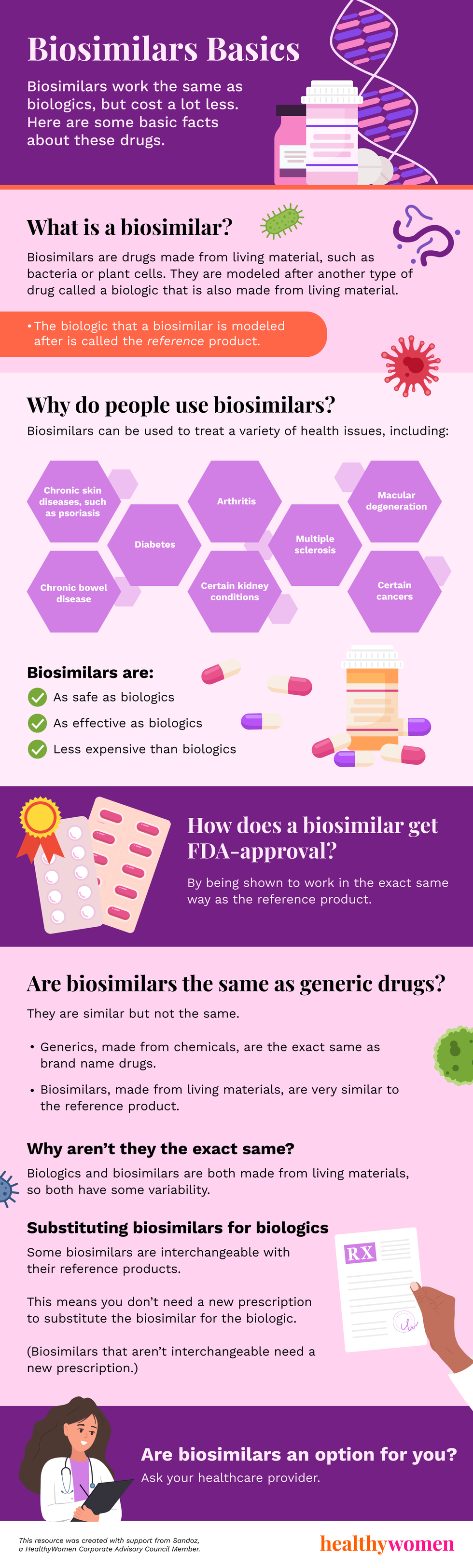
What is a biosimilar?
Biosimilars are drugs made from living material, such as bacteria or plant cells. They are modeled after another type of drug called a biologic that is also made from living material.
- The biologic that a biosimilar is modeled after is called the reference product.
Why do people use biosimilars?
Biosimilars can be used to treat a variety of health issues, including:
- Chronic skin diseases, such as psoriasis
- Chronic bowel disease
- Diabetes
- Arthritis
- Certain kidney conditions
- Multiple sclerosis
- Macular degeneration
- Certain cancers
Biosimilars are:
As safe as biologics
As effective as biologics
Less expensive than biologics
How does a biosimilar get FDA-approval?
By being shown to work in the exact same way as the reference product.
Are biosimilars the same as generic drugs?
They are similar but not the same.
Generics, made from chemicals, are the exact same as brand name drugs.
Biosimilars, made from living materials, are very similar to the reference product.
Why aren’t they the exact same?
Biologics and biosimilars are both made from living materials, so both have some variability.
Rx
Some biosimilars are interchangeable with their reference products.
This means you don’t need a new prescription to substitute the biosimilar for the biologic.
(Biosimilars that aren’t interchangeable need a new prescription.)
Are biosimilars an option for you? Ask your healthcare provider.
This resource was created with support from Sandoz, a HealthyWomen Corporate Advisory Council Member.
- How Are Biosimilars Made? ›
- Biosimilars 101 ›
- Biologics, Biosimilars and Generics: What’s the Difference? ›
- Questions to Ask Your Healthcare Provider About Biosimilars ›
- Clinically Speaking: What Are Biosimilars? ›