As told to Jacquelyne Froeber
September 25, 2024, is World Dense Breast Day.
After the mammogram, I sat in the waiting room wearing that god-awful paper gown.
The lump surprised me. It was big enough for me to feel during a self-exam, about the width of a fingertip. But I wasn’t too worried — I’d had my annual mammogram two months earlier and everything came back “normal.” Plus, I ate healthy, exercised and did my self-exams between mammograms. I thought I was doing everything right.
The technician finally returned, and I glanced at my watch thinking I would head to work as soon as she confirmed all was OK. “We didn’t see anything,” she said.
I thought she came back into the wrong room. “I’m the lady with a lump so big I can feel it,” I said. “Oh,” she looked at the chart. “You have dense breasts. That’s going to be a very hard find for us.”
“Wait, what?” I sat back confused. Dense breasts? “What does that mean?”
I was then sent for an ultrasound because what I felt did not show up on the mammogram.
The lump clearly showed up on ultrasound and on the MRI that followed, though. I had breast cancer. And because it was missed year after year on my mammogram, it was no longer early stage.
Finding out that I had breast cancer, dense breasts, and that the cancer was missed because of dense breast tissue — all within one afternoon — left me speechless.
When I got home, I researched “dense breasts.” I learned that 4 out of 10 women of mammography age have dense breasts and that dense breast tissue can hide cancer on a mammogram. I couldn’t quite grasp that no one had felt the need to share this with me at any of my previous mammograms. Shouldn’t the 40% of women with dense breasts be aware of this? Surely every patient should understand the realistic limitations of a test in their circumstances.
In the aftermath of the diagnosis, there was no time to process this. My focus was on the surgeries, treatments and navigating insurance issues. Because of the size and advanced stage of the cancer, my treatment involved eight surgeries, eight rounds of chemotherapy and 30 rounds of radiation after the cancer came back.
In 2010, with the bulk of the treatment behind me, I caught my breath and began researching dense breasts and cancer detection. Like me, most women I spoke to had no clue if they had dense breasts or not.
When a woman has a mammogram, her breast density is rated into one of four categories. I was shocked to learn that in women with the densest breasts, cancer will be missed about 50% of the time. A coin toss.
Certainly if I’d known this, I would’ve asked about other tests. The fact that I didn’t know effectively denied me the opportunity for an early-stage diagnosis.
In my research, I learned that, thanks to the work of a patient in Connecticut, the state had a law requiring that women receive a general notification about breast density after their mammogram. It had been a long time since 10th grade civics class, so I Googled, “How do you pass a law?” and got to work.
I reached out to my state senator in New York to ask if he would consider sponsoring the state legislation and he agreed. The comprehensive bill was drafted to include information all women should know: that she has dense breasts, that it can hide cancer, can increase her risk of developing breast cancer and that a woman with dense breasts should speak to her provider about additional screening tests after her mammogram.
I devoted all my time and energy to get state legislators to support the bill and spent many days in the state capitol visiting offices, making phone calls and sending emails.
In a win for New York women, the bill passed both the New York State Senate and Assembly unanimously, and was later signed into law. New York’s “density inform” law became the first to require that women with dense breasts be told specifically and unambiguously “Your breast tissue is dense.” Many other states used the New York bill as a template for their own laws.
While this was a huge step forward in the protection of New York women, my situation was not unique to my state. There were women all over the country going for annual mammograms, yet a hidden cancer was growing undetected in their dense tissue — and a later stage cancer was the result. Some of these patient advocates fought for their state legislation while in chemo chairs, getting infusions, typing to their legislators to request sponsorship of a bill.
Patient advocates were successful and there was a growing number of state “inform” bills, but they varied widely in the information each provided to women. Certainly, all U.S. women deserved the same actionable information and opportunity for an early stage diagnosis. A single national “dense breast” reporting standard was needed.
A national reporting standard could have been accomplished by federal legislation or federal regulation. I initiated efforts on both. I reached out to my representative in Congress, and he agreed to draft a federal bill that would standardize the information women got when they were notified that they had dense breasts.
The next step was the FDA. The letter women get after their mammogram is federally mandated by the FDA. So I wrote to them and asked them to consider a requirement to include information about a patient’s breast density in that letter.
To my relief, the FDA agreed to add the topic to the agenda of their next meeting of the National Mammography Quality Assurance Advisory Committee in 2011. I was invited to testify at the meeting and was joined by fellow patient advocates. Our message was clear: Every woman should be told about their own breast density and every woman deserves the same level of information regardless of her ZIP code.
The FDA advisory committee agreed.
In the years following the meeting, I continued to correspond with the FDA on the need for a single reporting standard for all U.S. women.
I’ve also continued to mentor advocates around the country working on legislation. And to address the new patient and healthcare provider conversations that begin with the question, “I have dense breasts, now what?” in 2015 I co-founded the website DenseBreast-info.org. The website is now the world’s leading online resource on the topic, is medically sourced and provides education to both people with dense breast tissue and healthcare providers.
Finally, earlier this month, more than 13 years after first testifying in front of the FDA, the federal breast density notification rule went into effect.
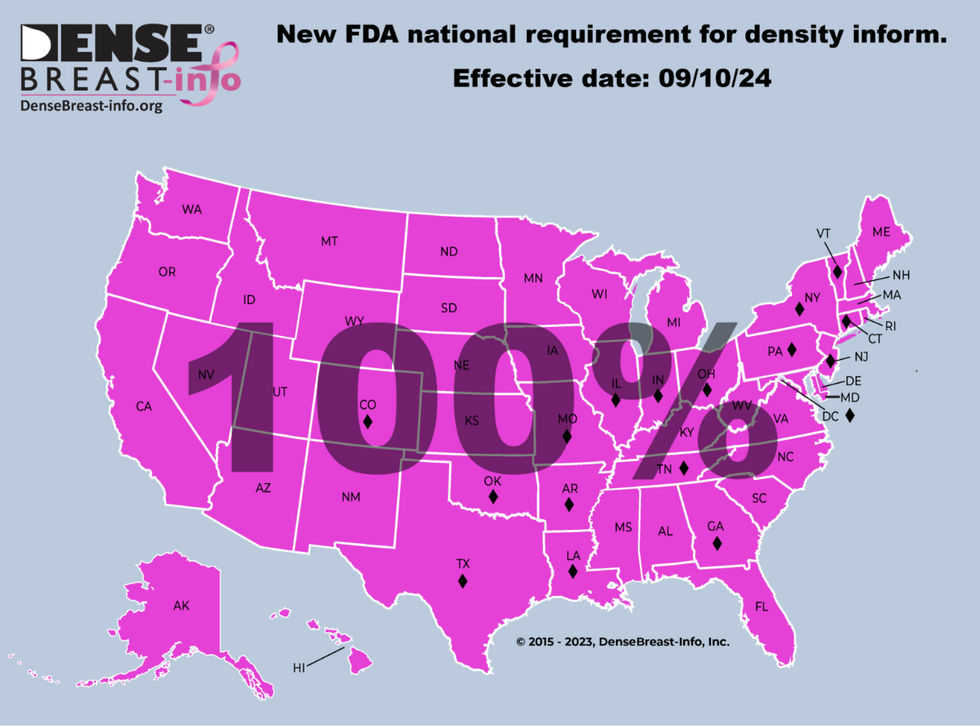
Reaching the finish line for a national “dense breast” reporting standard is bittersweet. I’m grateful, of course. I know this information will save lives. But there are so many women who fought for their own state laws that are no longer with us to join me in an exhale of relief.
So while we all share a hallelujah that this has finally come to pass, to quote Leonard Cohen, for some of us, “It’s a cold and broken hallelujah.”
But a hallelujah it is.
Have a Real Women, Real Stories of your own you want to share? Let us know.
Our Real Women, Real Stories are the authentic experiences of real-life women. The views, opinions and experiences shared in these stories are not endorsed by HealthyWomen and do not necessarily reflect the official policy or position of HealthyWomen.
- I Was Searching for a Miracle Cure for My Breast Cancer. I Found Hope Instead. ›
- How I Got My Sexual Groove Back After Surviving Breast Cancer ›
- After I Was Diagnosed with Triple Negative Breast Cancer, I Had Another Shock ›
- With Metastatic Breast Cancer, I Can’t Control Much — But That Only Makes Me Fight Harder For a Cure ›
- Real Women, Real Stories: Breast Cancer ›
- What You Should Know About Dense Breasts - HealthyWomen ›
- Lo que deberías saber sobre las mamas densas - HealthyWomen ›






